Building blocks for understanding
The periodic table - AQA Synergy
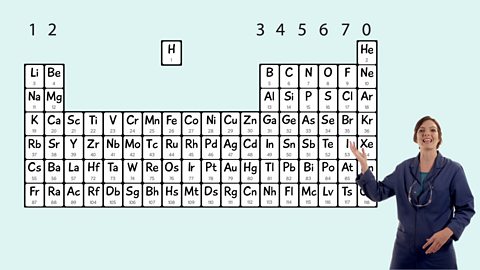
Mendeleev made an early periodic table. In the modern periodic table, elements are in order of atomic number in periods and groups. Electronic structures model how electrons are arranged in atoms.

Groups in the periodic table - AQA Synergy
Elements in the same group of the periodic table show trends in physical properties, such as boiling point. They have the same number of electrons in their outer shell, so they are similar in their chemical properties.

Chemical equations - AQA Synergy
Chemists use symbols and formulae to represent elements and compounds. Word equations and balanced chemical equations represent the changes that happen in chemical reactions.
Calculations in chemistry - AQA Synergy
Relative formula masses can be calculated and used in conservation of mass calculations. Calculations can be carried out to find out concentrations of solutions.

Sample questions - Building blocks for understanding - AQA Synergy
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription