Let's learn about batteries and how to make one.
Watch and learn
Video
Find out more about how we use batteries and what they are made from.
Oh, oh, the lights have stopped working. Looks like they are out of batteries.
Title: Making batteries
If something is battery powered, you don’t need to plug into an electrical socket.We use batteries in loads of everyday thing, like toys and games, TV remote controls, and torches.
Batteries are make from chemicals and metals that combine to make electrical energy. The chemicals inside a battery can make you very sick, but the hard outside shell keeps us safe.
The batteries inside a TV remote control are made up of cells. A cell contains two pieces of metal separated by a chemical that reacts with the metal, which generates the electrical energy.
When we add lots of cells together, they can produce more electrical energy, which flows when the battery is part of a complete electrical circuit.
We can actually make batteries from everyday household materials. For example, a lemon!
We also need two different types of metal and some copper wire. The wire is a conductive material that allows electrical energy to flow through it.
Roll the lemon so that the juice flows more easily inside. Push the zinc nail and the copper wire in. Be careful they don’t touch.
A voltmeter measures electrical energy. We can see on this voltmeter that we are making electrical energy from the lemon!
By combining lots of lemons (which are cells) together we can make a battery which will produce more electrical energy. A lemon battery!
Now, to power up all our lights! Well, maybe just one lightbulb.
Looks like we are going to need a lot more lemons.
Why do we use batteries?
Batteries provide a convenient, moveable source of electricity.
They are an essential part of most of our lives, from TV remote controls to toys and mobile phones to watches.
Can you make a list of all the things you use daily that have a battery?
There are lots of different types of batteries:
- different sizes
- different shapes
- different capacities (how much energy they can hold)
- non-rechargeable and rechargeable
- made from different materials
Here are some examples:

Image caption, Small batteries in watches
Watches don't need much power and need to be small and light, so they use very small, low-capacity batteries.
Image caption, Single-use batteries in toys
Many toys use small batteries that only need a small capacity. They are often light, single-use batteries.
Image caption, Rechargeable batteries in laptops
Laptop batteries are large, powerful and rechargeable. They are kept flat so that the laptop can be kept slim.
Image caption, Large batteries in cars
Car batteries are large rechargeable batteries with a big capacity.
1 of 4
How do batteries produce electricity?
Most of the electricity we use is generated by movement.
For example:
wind turns the blades of a wind turbine
steam from burning fossil fuels turns a turbine
water from a river turns a wheel
the movement of the tide moves a generator
But in a battery, electricity is produced in a completely different way.
A battery is made up of a series of cells stacked together. These contain chemicals that react and produce electricity when they are connected in a circuit.
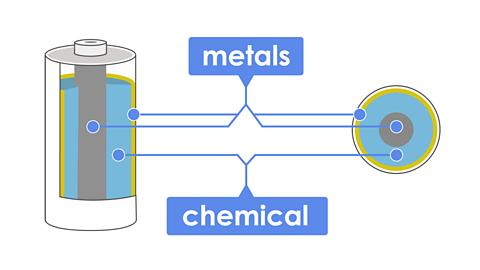
A cellThe single unit of a battery. It is made up of two different materials separated by a reactive chemical. is made up of:
two electrodes, each made from a different metal
these are separated by a chemical electrolyte (usually an acid and alkaliTypes of chemicals. Some are used in batteries because they react with the metals in a cell, producing electricity. Acids and alkalis can be dangerous.)
when the electrodes are connected a circuit is made. A chemical reaction causes electricity to from from one metal to the other and back through the electrolyte. (chemical energy is converted to electrical energy)
An individual cell doesn’t produce much electricity, but lots of them stacked together can power almost any device we need.

Be careful: The chemicals inside a battery can be very dangerous so they are encased in a hard shell to protect people. This is why you should never damage a battery or throw them in the bin where they might get broken and cause their chemicals to leak out.
Do batteries harm the environment?
Batteries have a negative impact on the environment.
Mining for the materials to make them causes damage to the environments in which they are found. Also, disposing of used batteries incorrectly releases dangerous chemicals back into the environment.

It is important to recycle batteries when you have finished with them (there are many places to recycle used batteries, for example, at supermarkets).
Consider using rechargeable batteries wherever possible to help reduce the impact on the environment.

What are batteries and energy stores? revision-guideWhat are batteries and energy stores?
Learn how batteries store energy and how they can help make our energy use more sustainable.

Metal and sustainability. revision-guideMetal and sustainability
Learn about metals, how they are produced and why we use them. Find out how they affect the environment, and how we can reduce, reuse and recycle metals to live more sustainably.

Activity

Make your own lemon battery!
Lemon juice is an acid and can be used as the electrolyte for a battery. Try it yourself!
You will need:
lemons
zinc nails
copper coins
electrical circuit wires with crocodile clips
an LED
Watch the video to find out what to do:
Video
Step-by-step instructions
Click through the slideshow for simple instructions on making your own lemon battery.

Image caption, 1. Roll a lemon.
This breaks up all the small sacs of lemon juice inside. Lemon juice is acidic and is going to be the chemical electrolyte that forms part of the electrical circuit.circuit.
Image caption, 2. Put the zinc and copper into a lemon.
At one end of the lemon insert the zinc about 2cm through the skin. At the opposite end inset the copper in the same way. These are called the electrodes and it is important that they don’t touch each other.
Image caption, 3. Connect the wire.
Connect a wire to each of the electrodes and connect to the voltmeter. Set the voltmeter to its most sensitive setting and you will be able to measure a small amount of electricity being produced. This is one cell: two pieces of different metal separated by a chemical (in this case the acidic lemon juice). The electrodes need to be made of different metals so that there is a chemical difference between them.
Image caption, 4. Repeat with two more lemons to create a battery.
We need more than one lemon cell to make a more powerful battiery. Repeat the previous steps with at least two more lemons. Connect the zinc in one to the copper in the next and so on (don’t connect zinc to zinc or copper to copper) before using wires to connect the battery to the voltmeter again. You should get a much bigger reading this time. Now take the voltmeter out of the circuit and replace with an LED. Remember: you have to connect the LED the correct way round - if it doesn’t work turn it round in the circuit.
Image caption, 5. Test it!
The LED should now light up, showing you have built a useful lemon battery.
1 of 5

Sorry, something went wrongCheck your connection, refresh the page and try again. – A device, made up of lots of cells, that makes electricity from a chemical reaction.
Sorry, something went wrongCheck your connection, refresh the page and try again. – The single unit of a battery. It is made of two different metals separated by a chemical.
Sorry, something went wrongCheck your connection, refresh the page and try again. – The chemical that connects the electrodes in a cell or battery. Electricity can flow through electrolyte, just like it flows through wires or metal.
Sorry, something went wrongCheck your connection, refresh the page and try again. – A part at each end of a battery. Electricity travels easily through them.
Quiz
More on Electricity
Find out more by working through a topic
- count3 of 8

- count4 of 8
- count5 of 8
