What's in my water?

Mouth-watering!
Water is incredible. All the things we love, from flowers to ice cream, need water in one way or another. But you might think that, on its own, water is a bit boring. Well â think again!
All water, from the water that comes out of our taps to all the rivers and lakes around us, contains chemicals called minerals.
While all tap water in the UK is safe to drink, not all water is the same. Depending on where we live, it can contain different types and amounts of those minerals. So letâs find out more.
In association with
Kingfisher Education Services

What is a mineral?
A mineral is a natural chemical thatâs often found dissolved in the water. A mineral can be an element (something that cannot be separated into a simpler substance) or a compound.
You will have heard of some of them like calcium, sodium and fluoride â one of the most common toothpaste ingredients. But others might be a bit more unusual, like manganese, potassium, and iodine.
You can see lists of minerals on the labels of bottled water, and the company that supplies water to your home should have information about whatâs in your tap water on their website.
Common minerals found in UK water are calcium, sodium, aluminium, chlorine, copper, iron, lead, magnesium, manganese and nitrogen, and compounds made from them.

How do minerals get into my water?
Minerals mainly get into water when the rain passes over rocks on its way through streams and rivers. Each time this happens, a little bit of the rock is dissolved into the water. Rocks all contain different minerals depending on the type of rock and when it was formed.
For example, if you live in an area with a lot of limestone or chalk rocks, like Derbyshire or Kent in England, then your water will contain higher amounts of a mineral called calcium carbonate, because thatâs what those rocks are made of.
If you live in an area of sandstone or granite like Grampian or the Lothians in Scotland, then youâll have a much lower level of calcium carbonate, because those rocks contain very little of it.
Try this!
Some minerals dissolve very easily into water, and some are very hard to dissolve. You can see this for yourself if you put a teaspoon of table salt (sodium chloride) into a large glass of water. Count how many stirs it takes for the salt to vanish. Now refill the glass and try it with a teaspoon of sand. Which one disappears more easily?

Are minerals good or bad?
Most of the minerals found in our water can be helpful to us and to other animals.
Calcium carbonate, for example, is used by the human body to keep bones and teeth healthy. Creatures that live in rivers and lakes also need the minerals in the water.
Invertebrates (creatures without backbones, like snails and water beetles) need calcium carbonate to make their exoskeletons, a hard outer skin, and shells. So in areas where there is less of that mineral in the water and substrate, for example at the bottom of a river, there will be fewer creatures with exoskeletons or shells, and more soft-bodied creatures such as worms.

What are âhardâ water and âsoftâ water?
The hardness of water is a measure of how much of certain minerals are present.
The main minerals that make water hard are calcium and magnesium, and compounds that contain them (like calcium carbonate mentioned above).
Hard water is good for bones and teeth, but not very good for washing machines and dishwashers, as the minerals come out of the hot water and coat the inside of the machines. Soft water is good for making soap bubbles but carries less bone-building minerals in it.
Try this!
To test whether youâve got hard or soft water at home is very easy. Take an empty drinks bottle and fill it half full of tap water. Put five drops of washing-up liquid into it then screw the top on tightly. Now the fun part â give it a shake for 30 seconds â as hard as you can! If you end up with lots of little bubbles in the bottle, youâve got soft water. If you end up with fewer, bigger bubbles then youâve got hard water.

How was our water formed?
Our oceans have not always been here on our planet.
The water within them is alien, which means it comes from outside Earth.
Water arrived here 4.5 billion years ago, many hundreds of millions of years after the Earth first took shape.
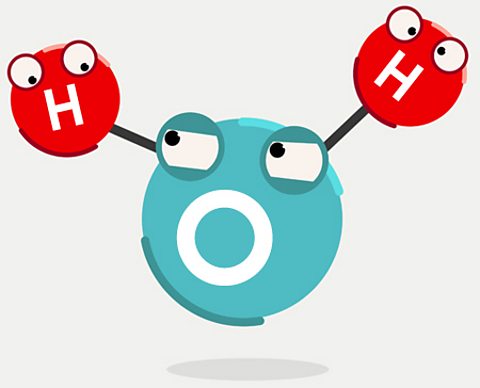
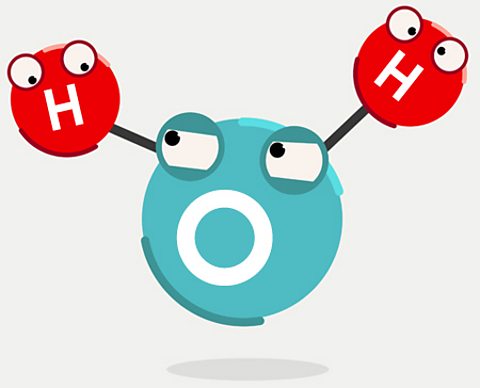
What is water made of?
To find the source of our oceans, letâs start with the raw ingredients: hydrogen and oxygen.
The hydrogen was made 13.7 billion years ago just a few minutes after the universe came into existence with the Big Bang. The oxygen atoms came millions of years later. As the universe expanded, huge clouds of hydrogen collapsed to form stars. As those stars shone, the hydrogen inside them fused into heavier elements such as carbon, nitrogen and oxygen.
Fast forward to around 5 billion years ago. Our Sun was being born from a cloud of collapsing hydrogen gas. Near that inferno, atoms of hydrogen and oxygen floated around in the vacuum of space. Occasionally both types of atoms might bump into tiny grains of dust, bits of carbon or silicon left over when previous stars died and exploded. The hydrogen and oxygen sometimes formed chemical bonds on these grains, to make H2O. All our water was created in space, on dust grains like this.
How did the Earth get its oceans?
Back when the Earth formed, around 4.5 billion years ago, the surface was like a vision of hell - volcanic and bone dry.
Our oceansâ water arrived on asteroids and comets from space during a violent episode in our planetâs early history, called the Late Heavy Bombardment.
The asteroids and comets came from the edge of our Solar System. They were the leftovers of the vast clouds of dust and rocks that didnât quite make it into planets and which were banished far away from the Sun once our eight planets had finally formed.

Why should we follow the water?
Water is the chemical of life on our planet.
Every living thing needs water to survive and wherever there is water on Earth, life has evolved to use it.
By looking for water in space, weâre really looking for the first sign that a place might also contain life.
If we could send a space probe to sample the water on Enceladus or Europa, we might come across our first example of alien bacteria or another primitive type of life.
Weâve been looking for it among the stars for centuries.
But life might exist right now in our own Solar System, floating in some alien water, just waiting to be found
Space water!
Did you know that there is water on almost every planet or rock in our Solar System? Click on the image below and then explore the labels to find out more.
Birth of a snowflake
When cold air meets warm air, ice crystals form six-sided shapes around a speck of dust, getting larger and larger as they fall. Drag the cursor across the video to see a close-up of beautiful snowflakes forming as moist air cools.
You may have seen igloos, little dome-shaped houses made of snow, in cartoons or films. But you may not realise how ingenious they are! Click on the labels to find out about traditional igloos made by the people of Igloolik, Canada.
Has a dinosaur drunk my water?

How old is Earth's water?
The atoms in the water on Earth have been here since the beginning of the planet - theyâve been here for four billion years!
A typical drop of water lasts about nine days in the sky before falling back to Earth. Before that, it may have been inside a leaf, or in a puddle, or in the ocean, to then become part of a cloud and fall as rain.
It might sound incredible, but the stuff that makes up our water is the same stuff the dinosaurs drank 150 million years ago. So how does that work?

Recycle, recycle and recycle!
Whether youâre making coffee or watering a plant, the atoms in that water will have been used and will continue to be used indefinitely. Atoms of water are ârecycledâ via the water cycle. When it rains, the water falling drop-by-drop from the sky was often in the oceans just a few days before.
But these are also broken down by photosynthesis and put back together during respiration.
When you drink a glass of water, that water isnât 'used up'; some of it ends up in your body. And some of it ends up in the toilet, of course, but your townâs water treatment plant cleans it up, and it too ends up right back in a river or lake â and it can one day be used again to make tea or lemonade, or fall as rain and water a plant.
Are we drinking dinosaur wee?
So does this mean weâre drinking dinosaur wee?
Click on image below and then on the labels to find out more.
A magic thing
All day, every day, water is broken apart and put back together, it gets mixed with some things, and separated out again. A little dirty, it gets cleaned up, and itâs ready to go again.
Due to processes like photosynthesis and respiration the actual water molecules may not be the same, but the atoms they're made of, are.
However, it is entirely possible that water which passed through dinosaurs has passed through us without changing for hundreds of millions of years!