Atomic structure
Models of the atom - AQA
The idea of the atom as the building block of matter has developed over time. What was thought of as a single particle about 1 Ă 10âŸÂčâ° m across is now known to be a collection of smaller particles.
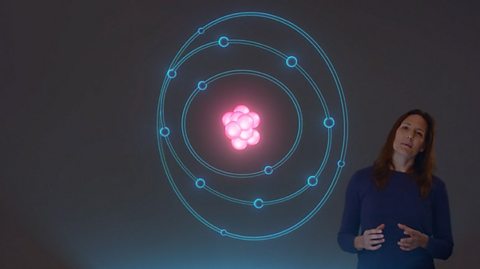
Atoms, isotopes and ions - AQA
Atoms are made up of protons, neutrons and electrons. Change the number of neutrons in an atom and it becomes an isotope, change the number of electrons, it becomes an ion.
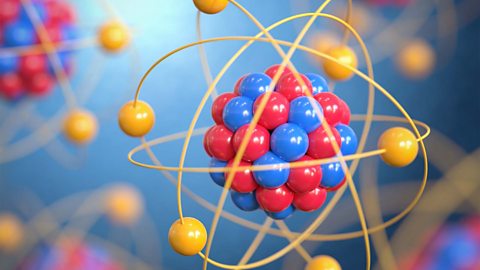
Radioactive decay - AQA
With the wrong number of neutrons, nuclei can fall apart. A nucleus will regain stability by emitting alpha or beta particles and then âcool downâ by emitting gamma radiation.

Uses and dangers of radiation - AQA
People are exposed to sources of radiation in all aspects of everyday life. Radioactive sources can be very useful but need handling carefully to ensure safety.

Nuclear fission and fusion - AQA
The nuclei of atoms contain a large amount of energy. Releasing this energy would free the world from having to use fossil fuels. There are two methods of doing this: fission and fusion.

Sample exam questions - atomic structure - AQA
Understanding how to approach exam questions helps to boost exam performance. Questions will include multiple choice, descriptions and explanations, using mathematical skills, and extended writing.

Links
- External linkExternal link
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link