Chemicals of the natural environment
Atomic structure of metals
Metals have characteristic properties which make them very useful. These can be explained using appropriate models of metallic structure and bonding.

Extracting metals with different reactivities
Metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other metal compounds. These reactions determine how it is extracted from its ore.

Electrolytes and electrolysis
Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. Metal ions and non-metal ions are attracted to opposite electrodes.

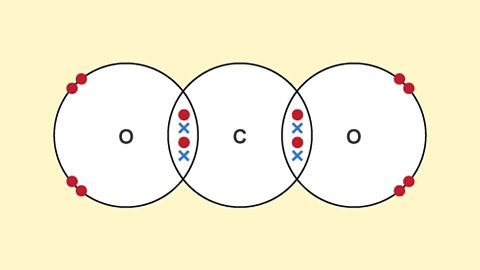
Covalent bonds
A covalent bond is a shared pair of electrons. Covalent bonding results in the formation of molecules. Simple molecular substances have low melting and boiling points, and do not conduct electricity.
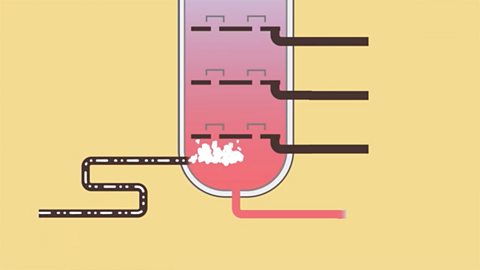
Crude oil
Crude oil is a finite resource. It can be separated into its useful fractions by fractional distillation. Some fractions undergo cracking to help meet demand for the smaller molecules.
Organic chemistry - OCR 21st Century
The alkanes form a homologous series of saturated hydrocarbons. Other homologous series are based on the same carbon chain but with the addition of a different functional group.

Sample exam questions - chemicals of the natural environment
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple-choice, structured, mathematical and practical questions.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link